at what distance is a 15 watt uv light effective to kill bacteria in water
- Inquiry commodity
- Open Access
- Published:
The efficacy of vacuum-ultraviolet calorie-free disinfection of some common environmental pathogens
BMC Infectious Diseases book 20, Article number:127 (2020) Cite this article
Abstract
Background
This study is to elucidate the disinfection effect of ozone producing low-force per unit area Hg vapor lamps confronting human pathogens. Ozone producing low-force per unit area Hg vapor lamps emit mainly 254 nm ultraviolet light C (UVC) with about ten% power of Vacuum-ultraviolet (VUV) light at 185 nm. The combination of UVC and VUV can inactivate airborne pathogens by disrupting the genetic materials or generation of reactive oxygen species, respectively. In this written report, inactivation of common leaner including Escherichia coli ATCC25922 (E. coli), Extended Spectrum Beta-Lactamase-producing Due east. coli (ESBL), Methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis (MTB), and that of influenza A viruses H1N1 and H3N2 nether the radiation from ozone producing depression-pressure Hg vapor lamps was examined. Log reduction values at different handling durations were determined.
Methods
In vitro tests were carried out. Diverse bacterium and virus suspensions were added onto nitrocellulose filter papers and subjected to the illumination from ozone producing low-pressure level Hg vapor lamps. The extents of pathogen inactivation at different illumination times were investigated by conducting a series of experiments with increasing duration of illumination. log10 reduction in CFU/ml and reduction at log10(TCIDl) were respectively measured for bacteria and viruses. The disinfection effectiveness of this blazon of lamps against the pathogens under the environment with a moderate barrier to light was therefore evaluated.
Results
Ozone producing low-pressure level Hg vapor lamp successfully inactivated these homo pathogens. Nonetheless, among these pathogens, disinfection of MTB required more than intense treatment. In the best tested situation, iii-log10 inactivation of pathogens can be achieved with ≤10 min of VUV handling except MTB which needed about 20 min. This demonstrated the loftier resistance against UV disinfection of MTB.
Conclusions
Following the criteria that valid germicidal results can be reflected with three-log10 inactivation for bacteria, iv-log10 inactivation for viruses and 5-log10 inactivation for MTB, most of the leaner required ≤10 min of VUV treatment, 20 min for the influenza viruses while MTB needed about 30 min VUV handling. This indicated that VUV calorie-free is an effective approach against different environmental microorganisms.
Groundwork
Indoor air quality (IAQ) has a meaning influence on health, comfort and well-being of building occupants. Information technology has been demonstrated that poor IAQ could jeopardize health and well-being, which in plough will affect the quality of work and ultimately lower the productivity of workers [1].
One major source of indoor air pollution is the presence of micro-organisms, which could cause even more serious problems than some organic and inorganic air contaminants. This is particularly more astounding in cases of inadequate ventilation, as the condensation in ventilation system tin act equally a breeding basis for harmful bacteria which are dispensed through the ventilation ducts. Environmental airborne bacteria such as Pseudomonas aeruginosa, Streptomyces albus, Bacillus subtilis and complex populations of micro-organisms within normal flora were all etiological agents to hypersensitivity pulmonary diseases. Several boosted infectious agents such as Legionella pneumophila and Mycobacterium tuberculosis (MTB) pose even more grave concerns to the IAQ, every bit these airborne pathogenic leaner are known to cause severe illness in humans. Meanwhile, viruses such as influenza virus were originally thought to exist only transmitted from person to person via aerosols of body fluids. However, in a contempo report conducted by Weistein et al. [2], the production of infectious droplet nuclei of diameter < 5 μm could remain suspended and disseminated past air current to infect a susceptible host. A adept and reliable disinfection system, therefore, is required to disinfect the airborne microorganisms in order to maintain practiced IAQ.
Adopting vacuum-UV (VUV) lamps, for case, the ozone producing depression-pressure Hg vapor lamps, tin exist an effective hateful of disinfecting the airborne microorganisms. Many existing infection command products utilize low force per unit area mercury vapor lamps as light source. This is a source of loftier energy photons with low cost. Recently, pulsed xenon light source technology emitting a broad spectrum (200-300 nm) of UV calorie-free is an emerging culling to depression force per unit area mercury vapor lamps that allows much faster surface disinfection because of the loftier acme power [three]. Even so, the pulsed nature of this technology would limit its use in continuous air disinfection system. Electrical discharge of low pressure mercury vapor mainly emits 254 nm ultraviolet light C (UVC) and 185 nm VUV light. However, existing products mainly utilize the lamps with doped quartz envelope that absorbs 185 nm photons to foreclose the formation of potentially unsafe ozone. Nevertheless, ozone is also a powerful disinfectant and the valuable disinfection opportunity of the 185 nm VUV light becomes waste heat.
Ozone is an issue that bothers on prophylactic if information technology remains in the output of an air treatment system. Still, ozone can be easily destroyed before leaving the air handling organization if proper catalyst is adopted [4, 5]. Too, some photocatalysts tin utilize and destroy ozone in addition to its photocatalytic action [6].
The 254 nm UVC lite adopted in conventional infection control products can disinfect the illuminated objects since the 254 nm radiation can disrupt the genetic materials of airborne pathogens and render them inviable [vii].VUV has an even stronger ionizing power than UVC lite and can generate high concentration reactive species such as ozone and OH radicals [7]. In other words, apart from directly illumination, VUV can inactivate bacterial growth by the radicals generated during VUV irradiation. Therefore, adopting VUV lamps can enhance the air disinfection capability of air cleaning systems. A previous report [4] conducted by Huang et al. demonstrated that 64% toluene removal with VUV irradiation lonely and the apply of photocatalyst enhanced the toluene removal from 64 to 82%. The experiment adopting UVC lamps and the utilise of photocatalyst removed merely 14% of toluene. The result demonstrated that VUV light could be an constructive measure out for chemical degradation in ventilation systems. When it comes to disinfection, all-encompassing research has been carried out on UVC light and effective devastation of both airborne [viii,9,x,11,12,thirteen,14,xv,16,17,18,19,20] and other homo pathogens [21,22,23,24,25,26,27,28,29] has been shown. Nonetheless, disinfection using VUV lite has attracted very niggling attending. This would be caused by the relative low prevalence of VUV lite sources. Kim et al. [thirty] found that the disinfection fourth dimension required to reach the same extent of inactivation of aerosolized MS2 bacteriophage, using depression pressure mercury vapor lamps with both 254 nm UVC and 185 nm VUV output was much shorter than the lamps with 254 nm UVC only. The disinfection time of ozone but (without UV) process at ozone concentrations equivalent to the ozone level generated past the mercury vapor lamps was also significantly faster than using lamps with 254 nm emission only. Besides, Huang et al. [four] reported the inactivation of E coli by low pressure mercury vapor lamps. Additionally, some researchers tested the disinfection of water with VUV low-cal and it was reported that the efficiency was quite low compared to disinfection with UVC light [31, 32]. The reason is due to the low penetration power of VUV calorie-free in h2o [33]. Moreover, the disinfection of man pathogens by VUV light was rarely reported. In our opinion, only Christofi et al. [34] reported the disinfection of the microbial films of three types of pathogenic bacteria using ozone producing low-pressure Hg vapor lamps. Therefore, the effect of VUV light against man pathogens is yet to be elucidated. In this study, we evaluated the germicidal effect of VUV light on common bacteria including Escherichia coli ATCC25922 (E. coli), Extended Spectrum Beta-Lactamase-producing Due east. coli (ESBL), Methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis (MTB), and that on influenza viruses H1N1 and H3N2. Flu viruses and MTB are inherent airborne pathogens while East. coli ATCC25922 is e'er the first indicator organism to monitor disinfection efficacy. The more drug resistant ESBL and MRSA were called every bit examples to monitor disinfection efficacy on human pathogens. Some suspensions of these bacteria and viruses were captivated into nitrocellulose filter papers during the experiments and the disinfection under the environment with a moderate barrier to light was evaluated.
Methods
UV irradiation
To evaluate the biocidal consequence of VUV low-cal, bacteria and viruses were irradiated with a pair of hot cathode depression pressure mercury vapor lamps. The lamps were 10 W, U-VIX brand, ZW10D15Y, ozone generating. The distance betwixt the calorie-free source and the microorganisms was approximately 5 cm and the UV intensities at 254 nm and 185 nm, respectively measured by a ZDZ-1 UV-C meter and an ILT1400 radiometer were 21 and 2.3 mW/cmii, respectively. To reduce the leakage of UV calorie-free and lamp-generated ozone to the surrounding, the lamps and the microorganisms under test were contained in a metal bedchamber during the experiments as shown in Fig. 1.
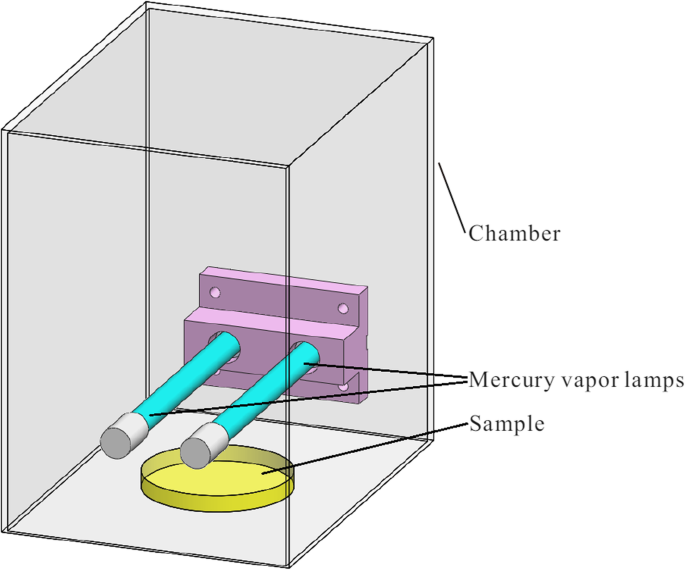
The VUV illumination experiment
Bacterial strains and inoculum preparation
Following procedures were used to prepare bacterial samples for UV irradiation experiments.
Escherichia coli ATCC25922 (E. coli), extended Spectrum Beta-Lactamase-producing E. coli (ESBL) and methicillin-resistant Staphylococcus aureus (MRSA)
Escherichia coli strain ATCC25922 (Eastward. coli), fully susceptible to most antibiotics, was purchased from American Type Civilization Collection (ATCC). Methicillin-resistant Staphylococcus aureus strain QC 5618 (MRSA) was provided equally a Proficiency Program of Central Public Health Laboratory, Colindale, UK. Extended Spectrum Beta-Lactamase-producing E. coli strain MM1604 (ESBL) was provided as a Proficiency Program of Central Public Health Laboratory Service, Department of Wellness, Hong Kong.
E. coli and MRSA were inoculated onto Mueller-Hinton agar (BD Bioscience, CA, USA) plates and incubated overnight at 37 °C to yield single colonies. Overnight cultures were prepared past inoculating single colonies of each bacterial strain into Brain Eye Infusion (BHI) broth (BD Bioscience, CA, U.s.a.). Bacterial intermission at early exponential phase was inoculated into BHI goop at 37 °C for 2 h. The concentration of the bacterial suspension was then visually adjusted to McFarland standard 0.5. Exam suspension was prepared past diluting the 0.5 McFarland standard inoculum by 10-fold and 100-fold. Actual bacterial count was calculated by dorsum titration of the inoculum break. Purity of MRSA was checked by ChromID® MRSA agar plate (BioMérieux SA, French republic) and the purities of E.coli and ESBL-producing E. coli were confirmed by MacConkey agar plate (Oxoid™, Thermo Scientific, Massachusetts, United States).
Mycobacterium tuberculosis (MTB)
MTB H37Rv (ATCC27294) was selected equally the model organism. Due to the infectivity and the risk of handling MTB, the experiments were conducted in the Biosafety Level-iii Laboratory of The University of Hong Kong.
MTB was first inoculated onto non-selective Middlebrook 7H11 agar (BD Bioscience, CA, USA) supplemented with 10% Oleic acid-Albumin-Dextrose-Catalase (OADC) and incubated at 37 °C with five% COii until single colonies were obtained. Mycobacterial colonies were resuspended into glass-bead Phospate-Buffered Saline with 0.1% Tween 80. Inoculum was vortexed for xxx s to homogenize the bacterial suspension. Bacterial concentration was and then adjusted to optical density at 600 nm = 0.15–0.17, which is equivalent to 0.5 McFarland standard. 2 examination suspensions were prepared, which were 0.5 McFarland standard inoculum and 10-fold diluted 0.v McFarland suspensions. Actual MTB count was calculated by back titration of the inoculum suspension on Middlebrook 7H11 agar. Purity of MTB was checked past culturing the inoculums on blood agar to ensure no fungal and bacterial contamination, and on non-selective Middlebrook 7H11 agar to ensure there was no contamination past nontuberculous mycobacteria.
Virus strains and cell lines
H1N1 and H3N2
Following procedures were used to prepare viral samples for UV irradiation experiments.
H1N1 was isolated from the first swine flu patient in Hong Kong in 2009 by the Section of Microbiology, The University of Hong Kong. H3N2, a seasonal flu in Hong Kong, was generously provided by Prof. H.L. Chen, Department of Microbiology, The University of Hong Kong. MDCK (Madin-Darby canine kidney) cell line provided past CDC, USA, was used to cultivate H1N1 and H3N2 viruses.
Both seasonal influenza A viruses were cultured in MDCK cells in MEM (GiBCO) supplemented with TPCK-trypsin (Sigma-Aldrich, MO, Usa). Virus-infected cells were harvested when virtually all MDCK cells exhibited cytopathic effects. Infected cells and the conditioned media underwent one freeze-thaw cycle to release viral particles. The suspension was then centrifuged at 3000 rpm for v min, and supernatant containing viral particles was collected. Tissue culture infective dose 50 (TCID50) was adamant in a 96-well tissue culture plate using Reed Muench method. Virus stock was stored at − eighty °C prior usage.
UV disinfection experiments
VUV disinfection experiments of Eastward. coli, ESBL and MRSA
To clarify the bactericidal effect of VUV light, two mL of bacterial interruption was added onto the nitrocellulose filter and irradiated by VUV for 2, v, 10 and fifteen min at a distance of 5 cm at 25 °C. This distance was selected based on the consideration of the time of disinfection and temperature rising of the agar during the course of experiments. Every bit each experiment was carried out within a Level-2 Biosafety Cabinet, the 2 mL added suspension was carefully adapted so that the filter remained moisted at the end of irradiation as dryness volition reduce the viable count recovered from the filter.
The illuminated bacterial suspension and the nitrocellulose filter were vigorously washed past 10 mL Phosphate-buffered saline (PBS). The intermission was then serially diluted with PBS from 100 to 10− iv, and 100 μL of each of the serially diluted bacterial suspensions was spread onto a Mueller-Hinton agar plate. Meanwhile, bacterial test suspensions without VUV illumination were spread onto Mueller Hinton agar to obtain the initial colony-forming units (CFU) before the use of VUV low-cal disinfection equally control.
All Mueller-Hinton agar plates were incubated overnight at 37 °C. The resultant CFU in each exam pause reflected the viable bacterial count later on different disinfection durations. The disinfection analysis was carried out in triplicate for each bacterial strain.
VUV disinfection experiments of Mycobacterium tuberculosis
To investigate the minimum time required by VUV low-cal for optimal MTB disinfection, test sets were used in which 2 mL concentration-adjusted MTB inoculums, added onto nitrocellulose filter papers, were illuminated by VUV for 10, 20, xxx and 45 min.
The illuminated bacterial suspension and nitrocellulose filter were vigorously done past 10 mL PBS, and the break was serially diluted (x0–10− iv). A total of 100 μL of each diluted bacterial interruption was spread onto selective Middlebrook 7H11 agar supplemented with 10% oleic albumin dextrose (OADC), 200,000 unit/L Polymyxin B, 50 mg/50 Carbenicillin, 10 mg/Fifty Amphotericin B and twenty mg/L Trimethoprim Lactate. Bacterial inoculum without VUV illumination was used as MTB growth control and to determine the original viable bacterial count. Each test ready was conducted in triplicate.
VUV disinfection experiments of flu viruses H1N1 and H3N2
To analyze the virucidal effect of VUV light, 2 mL virus samples at ~ one × 106 TCID50/mL were added onto nitrocellulose filter papers and irradiated by vacuum ultraviolet light (VUV) for 5, x, xv and xx min at an illumination distance of 5 cm at 25 °C. The illuminated viral break and nitrocellulose filter were vigorously washed, and the intermission was then serially diluted (100–10− 8) by Minimum Essential Medium (MEM) supplement with TPCK-trypsin. Each diluted sample was used to infect Madin-Darby Canine Kidney (MDCK) cells in the presence of TPCK-trypsin at 37 °C for iii days. The cease point of cytopathic effects (CPE) as minor, round and degeneration was recorded. Virus sample without VUV illumination was used to infect MDCK as positive control and to determine the original viral load. Each test was conducted in triplicate.
Information analysis
For bacteria, log10 reduction of viable bacterial count in CFU/mL was calculated by comparing command and postal service irradiation filters.
For influenza viruses, reductions at log10 (TCID50) was calculated similarly.
For each exam, outliers were removed past Dixon'due south Q test at 95% significance level. The resultant log10 reduction in CFU/ml of each bacterial strain and the resultant log10 reduction in TCID50 for each viral strain were plotted against disinfection durations, and error bars showing the data of the experiments that deviate from the corresponding mean value were also provided. MS Excel was used in all calculations and graph generation. A spreadsheet file containing raw information and intermediate calculations is provided in equally a supplementary data file.
Results
Escherichia coli ATCC25922 (E. coli)
Initial inoculum sizes for Due east. coli in 10-fold diluted and 100-fold diluted 0.5 McFarland standard inoculums across triplicate experiment sets, presented in the Additional file 1 as Expt. i and Expt. 2, were (1.9 ± 0.six) × 107 CFU/mL and (2.4 ± 0.2) × 106 CFU/mL, respectively. At ten min VUV light disinfection, the device was able to produce at least half dozen-log10 reduction in viable bacterial count for 100-fold diluted 0.5 McFarland standard inoculum. Nonetheless, 10 min VUV light disinfection for 10-fold diluted 0.5 McFarland standard inoculum can only achieve a borderline to insufficient bactericidal activity with an boilerplate ii.4-log10 growth reduction and 99.57% inhibition of bacterial growth (Fig. 2a and b). The results suggested that VUV calorie-free disinfection is much more constructive against lower E. coli bacterial concentration. At 15 min disinfection, complete inhibition of bacterial growth was also observed in x-fold diluted 0.5 McFarland standard inoculum, resulting in at to the lowest degree 6-log10 growth reduction (Fig. 2a and b).
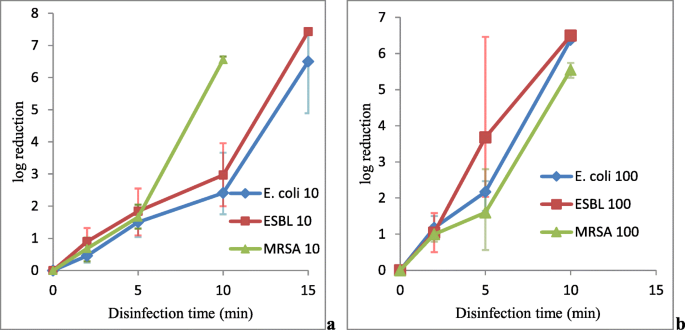
VUV light disinfection against E. coli, ESBL and MRSA. Both 10-fold (a) and 100-fold (b) diluted 0.5 McFarland standard inoculums of East. coli (denoted by Due east. coli with the dilution ratio behind), ESBL (denoted by ESBL with the dilution ratio behind) and MRSA (denoted by MRSA with the dilution ratio behind) were subjected to VUV light disinfection. The log10 (CFU/mL reduction) were plotted confronting the time of disinfection. Data were plotted as the means of triplicate biological replicates ±error
Extended Spectrum Beta-Lactamase-producing East. coli (ESBL)
Initial bacterial counts of ESBL for x-fold diluted and 100-fold diluted 0.5 McFarland standard inoculums beyond triple experimental sets, presented in the Additional file one as Expt. 3 and Expt. four, were (2.vii ± 0.3) × 107 CFU/mL and (three.2 ± 0.7) × 106 CFU/mL, respectively. It was observed that afterwards xv-min disinfection, both x-fold diluted and 100-fold diluted 0.5 McFarland standard inoculums were able to achieve complete inhibition of bacterial growth, resulting in at least 6-log10 growth reduction (Fig. 2a and b). However, at ten-min of disinfection time, although, the device was able to produce at least half dozen-log10 reduction of bacterial growth for the 100-fold diluted inoculum, VUV lite was only able to produce a borderline to insufficient bactericidal effect for the x-fold diluted 0.5 McFarland standard inoculum. The test just demonstrated an average of 2.96-log10 reduction with 99.63% growth inhibition. The results have demonstrated that VUV light is more effective against a lower concentration of ESBL.
Methicillin-resistant Staphylococcus aureus (MRSA)
Initial bacterial counts of MRSA for 10-fold diluted and 100-fold diluted 0.5 McFarland standard inoculums across triple experiment sets, presented in the Additional file i as Expt. v and Expt. 6, were (three.7 ± 0.9) × ten6 CFU/mL and (3.8 ± 1.seven) × 10five CFU/mL, respectively. At 10 min of VUV lite disinfection, the leaner of the 10-fold diluted and the 100-fold diluted 0.five McFarland standard inoculums were completely inhibited, resulting in at least 5-log10 growth reduction (Fig. 2a and b).
Mycobacterium tuberculosis (MTB)
As defined in previous sections, disinfection time against bacteria was considered sufficient when a minimum 3-log10 reduction of viable bacterial count was observed. For mycobactericidal activeness, a 5-log10 reduction in feasible bacterial load is required due to the highly infectious nature of MTB. In other words, a minimum of 5-log10 viable bacterial load would be required for a valid experimental set. The boilerplate bacterial concentration for McFarland standard 0.5 MTB inoculum was only (3–5) × x6 CFU/mL according to our previous experiments (information non shown). When the bacterial inoculum was diluted by 100-fold, the bacterial concentration would merely be effectually 104 CFU/mL. The bacterial load could be too low and it was incapable of illustrating 5-log10 growth reduction. The experiment was therefore conducted with a higher bacterial concentration and more detailed disinfection fourth dimension equally compared to the tests of other bacteria. 0.5 McFarland standard and 10-fold diluted 0.v McFarland standard inoculums were used and irradiated by VUV for 10, 20, 30 and 45 min. Initial bacterial counts for 0.v McFarland standard and the 10-fold diluted 0.five McFarland standard MTB inoculums were (4.iv ± i.seven) × x6 CFU/mL and (1.2 ± 0.2) × 105 CFU/mL, respectively, presented in the Additional file one as Expt. vii and Expt. 8.
Gradual reduction in bacterial count was observed with prolonged VUV disinfection time. Complete inhibition of bacterial growth was observed subsequently xxx min VUV low-cal disinfection. At twenty min VUV illumination, VUV light was able to produce an boilerplate of 4-log10 and iii.six-log10 reduction in 0.five McFarland standard and the 10-fold diluted 0.5 McFarland standard inoculums, respectively (Fig. 3).
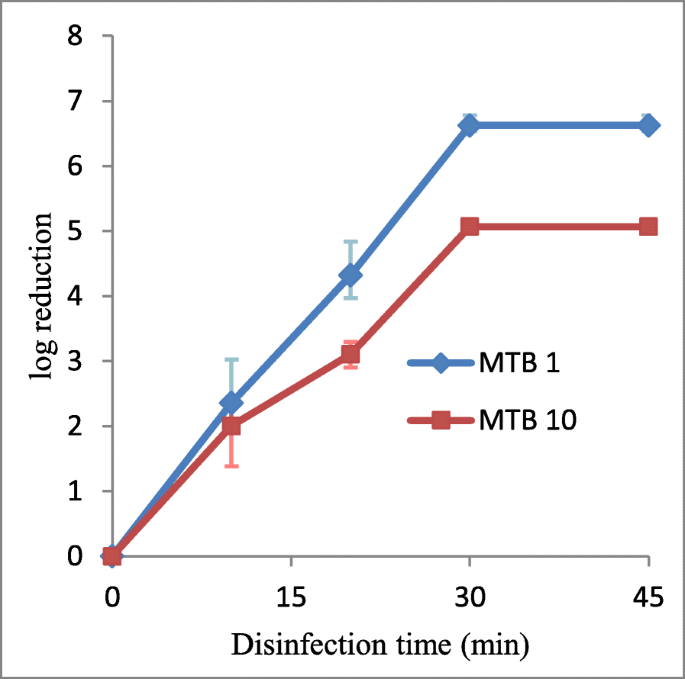
VUV calorie-free disinfection against MTB. The experimental sets were conducted on 0.5 McFarland standard inoculum (denoted past MTB 1) and 10-fold diluted 0.5 McFarland standard inoculum (denoted by MTB 10). The log10 (CFU/mL reduction) were plotted confronting the fourth dimension of disinfection. Information were plotted equally the means of triplicate biological replicates ±error
In the present study, we have demonstrated that VUV low-cal disinfection can attain consummate inactivation of MTB growth after 30 min disinfection regardless of the bacterial concentration. Meanwhile at 20 min, VUV light disinfection can only result in a minimum of 3-log10 reduction in bacterial count, which is much longer when compared to the E coli, ESBL and MRSA experiments described in previous sections. Previous studies [19, 35, 36] showed that mycobacterial species are generally more resistant to UV disinfection, but are subject to a amend disinfection effect under VUV light. Information technology seemed that VUV low-cal disinfection was less constructive against MTB at a lower bacterial concentration.
Influenza viruses H1N1 and H3N2
Meanwhile for viral disinfection, exam results were considered acceptable when the viral-induced cytotoxic effect is indistinguishable from test agent-induced cytotoxic furnishings. VUV light disinfection time against viruses would be considered sufficient when a minimum of 3-log10 reduction in viral-induced cytotoxicity in titer was accomplished. Therefore, the infectious viruses recovered from the positive controls must be ≥4-log10 for valid viricidal test results. To make up one's mind the disinfection efficacy of VUV low-cal confronting seasonal influenza viruses, two mutual influenza A viruses, H3N2 and H1N1, causing seasonal epidemics were used. In the current study, initial viral loads for both H1N1 and H3N2, presented in the Boosted file 1 as Expt. nine and Expt. 10, were 5.4 ± 0.four log10(TCIDl/mL) and v.1 ± 0.8 log10(TCID50/mL), respectively.
For samples with log10(TCIDl/mL) less than 1.5, the titer was treated every bit 0.five for log reduction calculation and graph plotting purpose.
At five min of illumination, VUV calorie-free tin inactivate H1N1 and H3N2 by 2.two- and 3.0-log10 folds viral load (TCID50), respectively (Fig. iv). When the VUV illumination time was extended to xx min, more than four-log10 reductions in TCID50 of both seasonal influenza A viruses were observed.
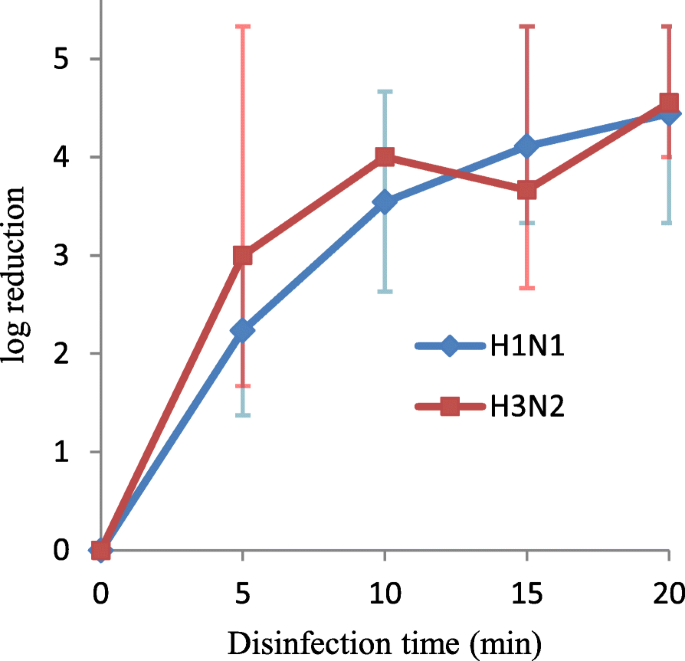
VUV calorie-free disinfection against H1N1 and H3N2 influenza A viruses. The log10 (TCIDfifty/mL reduction) was plotted confronting disinfection time
Word
Loftier-energy vacuum-UV light is efficient in disinfection. Similar to other UV disinfection mechanisms, direct illumination of VUV could result in the formation of new bonds between next nucleotides, causing photochemical damage on DNA strands and somewhen inactivating the replication of microorganisms.
In addition, the high-energy VUV could also atomic number 82 to the formation of both OH radicals and Othree, which diffuse into anywhere that is shielded from direct UV irradiation and inhibit the growth of microorganism. This explained the excellent bactericidal efficiency of VUV light disinfection even in the presence of the opaque nitrocellulose filter. Our result has further revealed the potential of VUV light to provide a thorough disinfection, even for dust particles and large aerosols contaminated with pathogens where direct UV illumination cannot penetrate.
In this study, we demonstrated that VUV light disinfection is effective against Escherichia coli, Extended Spectrum Beta-Lactamase-producing E. coli and Methicillin-resistant Staphylococcus aureus. For the best tested state of affairs, with the benchmark of three-log10 inactivation of bacteria, valid germicidal result can exist achieved with ≤10 min of VUV handling. Additionally, more than v-log10 reduction in viable plate count can be attained below 15 min of disinfection.
In the disinfection tests confronting seasonal influenza viruses H1N1 and H3N2, we also demonstrated that viral load could be effectively reduced by iv-log10 folds later 20 min VUV illumination and this also satisfied the criterion of valid germicidal effect. Additionally, more than 3-log10 reduction in viral load can be attained with < 10 min of handling.
Mycobacterium tuberculosis, on the other manus, required a more intense disinfection.
At xx min disinfection, VUV lite disinfection could only result in a three-log10 reduction in viable plate count. This is insufficient according to our 5-log10 reduction criterion for mycobacterial disinfection. It was just after 30 min of disinfection that the required 5-log10 reduction of Mycobacterium tuberculosis feasible bacterial load could be achieved regardless of the bacterial concentration. This is concordant to previous studies [xix, 35, 36] where mycobacterial species were mostly more resistant to UV disinfection. This is probably accounted past the thicker lipid cell wall in Mycobacterium species.
The tested variations in concentrations of bacteria did not manifest a trend in the rate of inactivation. For E. coli and ESBL, higher bacterial concentration resulted in lower rates of inactivation. Experiments with MTB showed a different tendency. Notwithstanding, no obvious trend was showed in the experiments with MRSA.
From literature, diverse research teams reported the UV dosages required attaining 99.9% (iii-log) inactivation of various bacteria or viruses under light from depression pressure mercury vapour lamps. For example, the UV dosages in mJ/cmtwo for 3-log inactivation of T7 phage, Eastward coli., Staphylococcus aureus, Mycobacterium avium and Mycobacterium phlei are 10 [37], v [37], 9 [34], xviii [20] and 158 [34], respectively. Most of their experiments were conducted with bacteria and viruses most unprotected. In our experiment, attaining 3-log inactivation typically required 10 min. Considering that our equipment provided 21 and ii.3 mW/cm2 light ability at 254 nm and 185 nm, and the total UV power is ~ 23 mW/cm2. The UV dosage of 10 min illumination is ~ 14,000 mJ/cm2, far higher than the usual values. This could be the event of our testing status created past loading the suspended bacteria or viruses onto nitrocellulose filter paper. Some bacteria were actually protected from direct UV light by the shading effect of filter newspaper which is unlike from the testing setup in the literature.
In club to provide sufficient disinfection against all the microorganisms we included in this report, we suggested the use of Mycobacterium reduction as a criterion test for future disinfection musical instrument designs that incorporates the VUV light arrangement.
Although, the disinfection under the environment with a moderate barrier to light was successful, there are limitations in the present written report. The current airplane pilot written report on the disinfection efficacy of VUV light disinfection was conducted in laboratory-controlled atmospheric condition. For instance, due to condom consideration, device type testing on aerosolized bacteria and viruses is not possible. All bacterial and viral inoculums were prepared in liquid break and illuminated by VUV on a Petri dish, which differed from actual environmental settings.
Conclusion
Airborne pathogens are important indoor air quality concerns. A good and reliable disinfection organization is a must to maintain good indoor air quality. Vacuum-UV lamps with ozone production were establish to exist effective for inactivating various human pathogens. With the best tested state of affairs, three-log10 inactivation of Escherichia coli, Extended Spectrum Beta-Lactamase-producing E. coli, Methicillin-resistant Staphylococcus aureus and seasonal influenza viruses can be achieved with ≤10 min of VUV treatment except Mycobacterium tuberculosis which needed near 20 min. This demonstrated the loftier resistance against UV disinfection of MTB. Valid germicidal results, reflected with 3-log10 inactivation for bacteria, 4-log10 inactivation for viruses and 5-log10 inactivation for MTB, can exist obtained with all tested pathogens. The duration of VUV treatment required for valid germicidal result of most of the bacteria was ≤x min while MTB needed about 30 min. 20 min was acceptable for the influenza viruses. This indicated that VUV light is an effective approach confronting dissimilar environmental and pathogenic microorganisms, and can potentially be used for air-purifying units in futurity ventilation systems.
Availability of data and materials
All information supporting the findings in this report are contained within the supplementary information files.
Abbreviations
- ATCC:
-
American type civilization collection
- BHI:
-
Brain heart infusion
- CFU:
-
Colony-forming units
- CPE:
-
Cytopathic issue
- E. coli :
-
Escherichia coli
- ESBL:
-
Extended spectrum beta-lactamase
- IAQ:
-
Indoor air quality
- MDCK:
-
Madin-Darby canine kidney
- MEM:
-
Minimum essential medium
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MTB:
-
Mycobacterium tuberculosis
- O3 :
-
Ozone
- OADC:
-
Oleic acid-albumin-dextrose-catalase
- OH:
-
Hydroxyl radical
- PBS:
-
Phosphate-buffered saline
- TCIDfifty :
-
Tissue culture infective dose 50
- TPCK:
-
6-(one-tosylamido-2-phenyl) ethyl chloromethyl ketone
- UV:
-
Ultraviolet
- UVC:
-
Ultraviolet C
- VUV:
-
Vacuum ultraviolet
References
-
Kosonen R, Tan F. The issue of perceived indoor air quality on productivity loss. Energy Buildings. 2004;36(x):981–6.
-
Weinstein RA, Bridges CB, Kuehnert MJ, Hall CB. Transmission of flu: implications for control in health care settings. Clin Infect Dis. 2003;37(8):1094–101.
-
Wang T, MacGregor Due south, Anderson J, Woolsey G. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005;39(xiii):2921–5.
-
Huang H, Leung DYC, Li G, Leung MK, Fu 10. Photocatalytic destruction of air pollutants with vacuum ultraviolet (VUV) irradiation. Catal Today. 2011;175(1):310–5.
-
Batakliev T, Georgiev V, Anachkov Chiliad, Rakovsky S. Ozone decomposition. Interdiscip Toxicol. 2014;7(two):47–59.
-
Wu K, Leung DYC, Zhang Y, Huang H, Xie R, Szeto West, Li F. Toluene deposition over Mn-TiO2/CeO2 composite catalyst under vacuum ultraviolet (VUV) irradiation. Chem Eng Sci. 2019;195:985–94.
-
Schalk S, Adam Five, Arnold East, Brieden G, Voronov A, Witzke H-D. UV-lamps for disinfection and advanced oxidation-lamp types, technologies and applications. IUVA News. 2005;8(one):32–seven.
-
Brickner Pw, Vincent RL, First M, Nardell Eastward, Murray 1000, Kaufman W. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: bioterrorism countermeasure. Public Wellness Rep. 2003;118(2):99.
-
Escombe AR, Moore DA, Gilman RH, Navincopa One thousand, Ticona Due east, Mitchell B, Noakes C, Martínez C, Sheen P, Ramirez R. Upper-room ultraviolet light and negative air ionization to forbid tuberculosis transmission. PLoS Med. 2009;6(three):e1000043.
-
Xu P, Peccia J, Fabian P, Martyny JW, Fennelly KP, Hernandez M, Miller SL. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-calibration studies. Atmos Environ. 2003;37(3):405–19.
-
Goldstein MA, Tauraso NM. Effect of formalin, β-propiolactone, merthiolate, and ultraviolet light upon influenza virus infectivity, chicken cell agglutination, hemagglutination, and antigenicity. Appl Environ Microbiol. 1970;nineteen(2):290–iv.
-
Walker CM, Ko Thou. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ Sci Technol. 2007;41(xv):5460–v.
-
Green CF, Scarpino PV. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ Eng Policy. 2001;3(ane):101–vii.
-
Peccia J, Werth HM, Miller S, Hernandez 1000. Effects of relative humidity on the ultraviolet induced inactivation of airborne bacteria. Droplets Sci Technol. 2001;35(3):728–40.
-
Chumpolbanchorn Yard, Suemanotham N, Siripara N, Puyati B, Chaichoune G. The effect of temperature and UV calorie-free on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure; 2006.
-
Hollaender A, Oliphant JW. The inactivating result of monochromatic ultraviolet radiations on influenza virus. J Bacteriol. 1944;48(4):447.
-
Lai Thousand-M. Using selective media to assess aerosolization harm and ultraviolet germicidal irradiation susceptibility of Serratia marcescens. Aerobiologia. 2005;21(3–four):173–9.
-
Nardell EA, Bucher SJ, Brickner Prisoner of war, Wang C, Vincent RL, Becan-McBride Yard, James MA, Michael M, Wright JD. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the tuberculosis ultraviolet shelter report. Public Health Rep. 2008;123(1):52–60.
-
Collins FM. Relative susceptibility of acrid-fast and not-acid-fast leaner to ultraviolet light. Appl Microbiol. 1971;21(3):411–3.
-
Shin G-A, Lee J-K, Freeman R, Cangelosi GA. Inactivation of Mycobacterium avium circuitous by UV irradiation. Appl Environ Microbiol. 2008;74(22):7067–9.
-
Rutala WA, Gergen MF, Weber DJ. Room decontamination with UV radiations. Infect Control Hosp Epidemiol. 2010;31(10):1025–nine.
-
Truman RW, Gillis TP. The outcome of ultraviolet light radiation on Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 2000;68(one):11–vii.
-
Mofidi AA, Baribeau H, Rochelle PA, De Leon R, Coffey BM, Light-green JF. Disinfection of Cryptosporidium parvum with polychromatic UV calorie-free. J-Am Water Works Assoc. 2001;93(6):95–109.
-
Yaun BR, Sumner SS, Eifert JD, Marcy JE. Response of Salmonella and Escherichia coli O157: H7 to UV energy. J Nutrient Prot. 2003;66(6):1071–three.
-
Zimmer J, Slawson R, Huck P. Inactivation and potential repair of Cryptosporidium parvum following low-and medium-force per unit area ultraviolet irradiation. H2o Res. 2003;37(fourteen):3517–23.
-
Jinadatha C, Villamaria FC, Restrepo MI, Ganachari-Mallappa N, Liao I-C, Stock EM, Copeland LA, Zeber JE. Is the pulsed xenon ultraviolet light no-touch disinfection system constructive on methicillin-resistant Staphylococcus aureus in the absence of manual cleaning? Am J Infect Control. 2015;43(eight):878–81.
-
Hassen A, Mahrouk M, Ouzari H, Cherif M, Boudabous A, Damelincourt JJ. UV disinfection of treated wastewater in a big-scale airplane pilot plant and inactivation of selected bacteria in a laboratory UV device. Bioresour Technol. 2000;74(ii):141–50.
-
Sommer R, Lhotsky M, Haider T, Cabaj A. UV inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli O157 and other pathogenic Escherichia coli strains in water. J Nutrient Prot. 2000;63(eight):1015–20.
-
Jinadatha C, Quezada R, Huber TW, Williams JB, Zeber JE, Copeland LA. Evaluation of a pulsed-xenon ultraviolet room disinfection device for touch on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect Dis. 2014;xiv(1):187.
-
Kim J, Jang J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci Technol. 2018;52(5):557–66.
-
Ramsay IA, Niedziela J-C, Ogden ID. The synergistic upshot of excimer and depression-pressure mercury lamps on the disinfection of flowing water. J Food Prot. 2000;63(eleven):1529–33.
-
Wang D, Oppenländer T, El-Din MG, Bolton JR. Comparison of the disinfection effects of vacuum-UV (VUV) and UV light on Bacillus subtilis spores in aqueous suspensions at 172, 222 and 254 nm. Photochem Photobiol. 2010;86(one):176–81.
-
Zoschke Thousand, Börnick H, Worch East. Vacuum-UV radiation at 185 nm in water treatment–a review. Water Res. 2014;52:131–45.
-
Christofi N, Misakyan M, Matafonova G, Barkhudarov E, Batoev V, Kossyi I, Sharp J. UV treatment of microorganisms on artificially-contaminated surfaces using excimer and microwave UV lamps. Chemosphere. 2008;73(5):717–22.
-
Peccia J, Hernandez M. UV-induced inactivation rates for airborne Mycobacterium bovis BCG. J Occup Environ Hyg. 2004;i(7):430–v.
-
Bohrerova Z, Linden KG. Assessment of Deoxyribonucleic acid impairment and repair in Mycobacterium terrae subsequently exposure to UV irradiation. J Appl Microbiol. 2006;101(5):995–1001.
-
Bohrerova Z, Shemer H, Lantis R, Impellitteri CA, Linden KG. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Res. 2008;42(12):2975–82.
Acknowledgements
Not applicative.
Funding
Funding was provided past the National Natural Science Foundation of Communist china (NSFC) and The Enquiry Grants Quango (RGC) of Hong Kong. The funding bodies were not involved in the preparation of the manuscript.
Author information
Affiliations
Contributions
WS was a major contributor in writing and editing the manuscript. WCY was responsible for the experimental investigation and assay. HH provided comments on the experiment setup and manuscript. DYCL coordinated the test and approved the final manuscript. All authors read and approved the final manuscript.
Respective author
Ethics declarations
Ethics blessing and consent to participate
Non applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher'south Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary data
Additional file 1.
This file contains all data supporting the findings in this study which is a spreadsheet file containing raw data and intermediate calculations.
Rights and permissions
Open up Access This commodity is distributed under the terms of the Creative Commons Attribution iv.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted utilise, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons license, and indicate if changes were fabricated. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/ane.0/) applies to the data fabricated available in this article, unless otherwise stated.
Reprints and Permissions
Most this article
Cite this commodity
Szeto, Due west., Yam, Westward.C., Huang, H. et al. The efficacy of vacuum-ultraviolet light disinfection of some mutual environmental pathogens. BMC Infect Dis 20, 127 (2020). https://doi.org/10.1186/s12879-020-4847-ix
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/ten.1186/s12879-020-4847-9
Keywords
- Disinfection
- Microorganism
- Ozone
- VUV
- IAQ
- Influenza
- Tuberculosis
- ESBL
- MRSA
Source: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-020-4847-9
0 Response to "at what distance is a 15 watt uv light effective to kill bacteria in water"
Post a Comment